- Products
- Oncohematology
- Antibodies
- Kits
- CAR T-cell
- Euroflow
- Single reagents
- Request info
- Resources and support
- Immunology
- Antibodies
- Single reagents
- Cross match determination (FCXM)
- FcεR1
- Ig subclasses
- Single reagents
- Kits
- TiMas, assessment of tissue macrophages
- Request info
- Resources and support
- Antibodies
- Exosomes
- Accesory reagents
- Software
- Oncohematology
- Services
- Peptide Production
- Design
- Modification
- Protein Services
- Expression and purification
- Freeze drying
- Monoclonal And Polyclonal Antibody Development
- Monoclonal
- Policlonal
- Specialized antibody services
- OEM/Bulk production
- Purification
- Conjugation
- Custom Exosome Services
- Isolation and purification
- Characterization
- Peptide Production
- Shop
- Support
- About Us
- Contact

Anti-SARS-CoV-2 Multiplex serology assay
One of the most sensitive and reliable SARS-CoV-2 assays available in the market.
Flow Cytometry SARS-CoV-2 multiantigen test
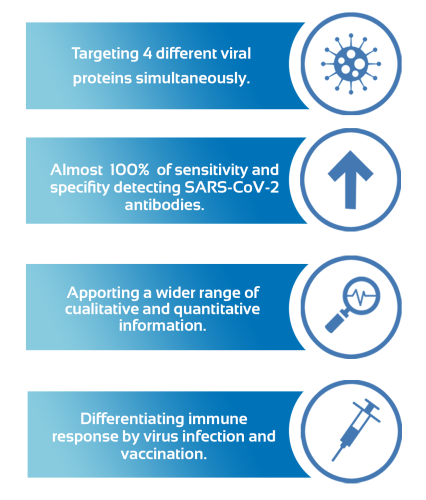
Simultaneously target four separate SARS-Cov-2 viral proteins and 3 immunoglobulins.
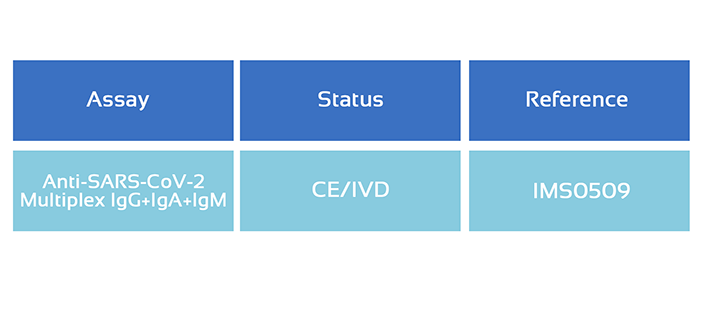
SARS-CoV-2 Multiplex IgG+IgA+IgM kit
Multiantigen IgG+IgA+IgM is a multiplex, microsphere-based, highly sensitive and specific assay that measure the presence or absence of antibodies against four different SARS-CoV-2 antigens simultaneously.

RBD
Detecting SARS-CoV-2 neutralizing antibodies.

Spike
Identifying immune response after vaccination.

Nucleocapsid
Identifying immune response to natural infection.

Mpro
Identifying immune response to viral replication.
Three reporter channels simultaneously
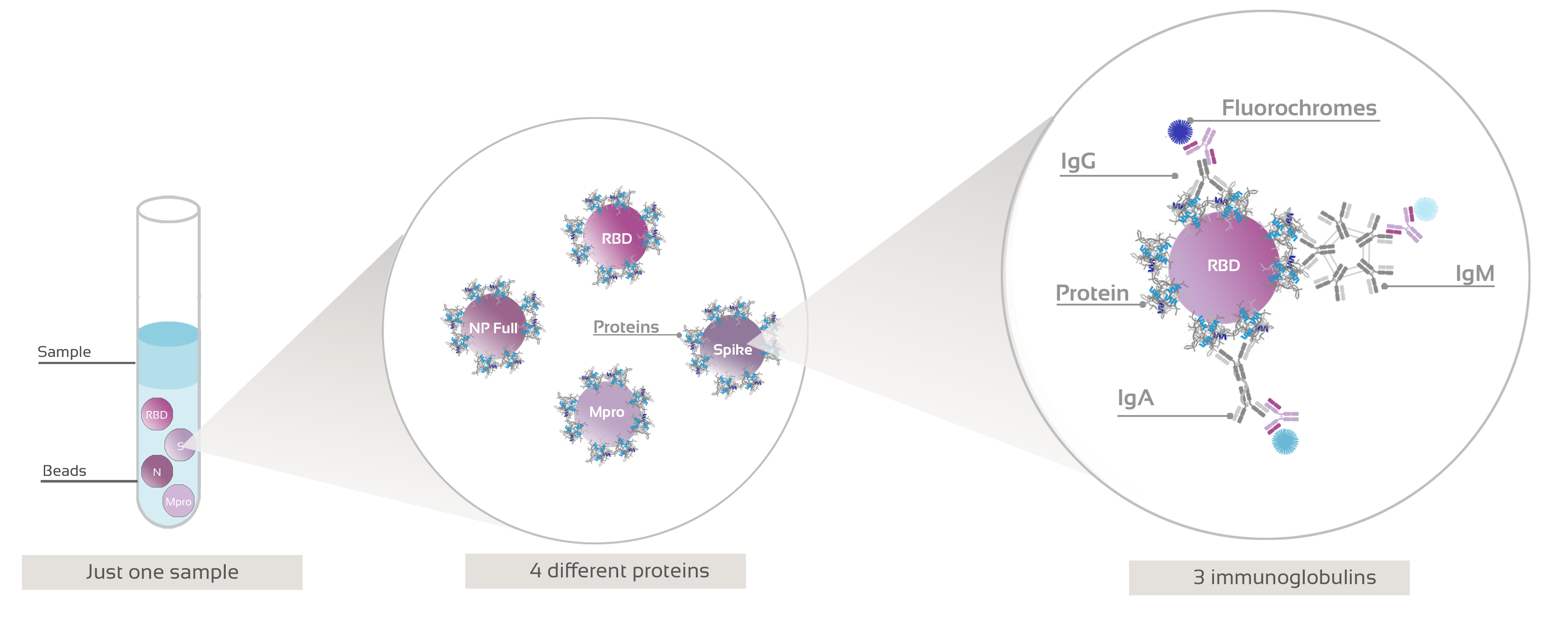
Figure 1: Graphical representation of Anti-SARS-CoV-2 flow cytometry bead-based assay procedure.
Differentiating immune response by virus and vaccination
This serological bead-based assay has demonstrated to provide a wider range of information about the immune system response to SARS-CoV-2 than any other assay in the market, and it is performed in just 2 hours.
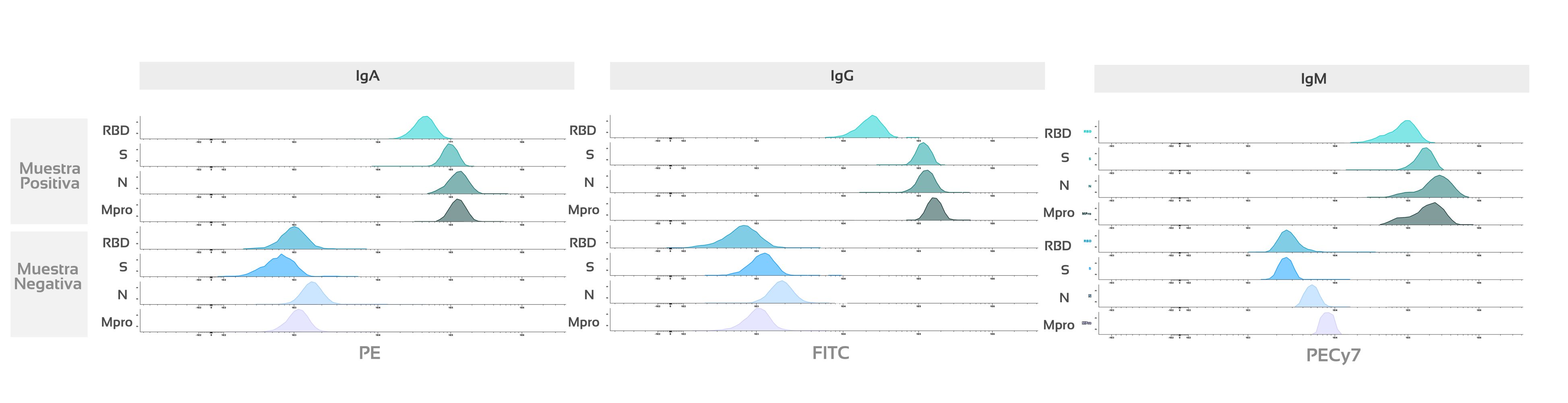
Figure 2: Histogram in cytometry comparing analysis results of positive vs. negative sample for 4 viral proteins: Mpro: Main virus protease or 3C-type protease (Mpro/3CLpro). | NP: Nucleocapsid protein (N). | S: Stable trimer of the spicule glycoprotein (S). | RBD: Receptor-binding domain (RBD) of S-glycoprotein) and 3 immunoglobulins (IgA, IgG, and IgM).
What this kit includes?
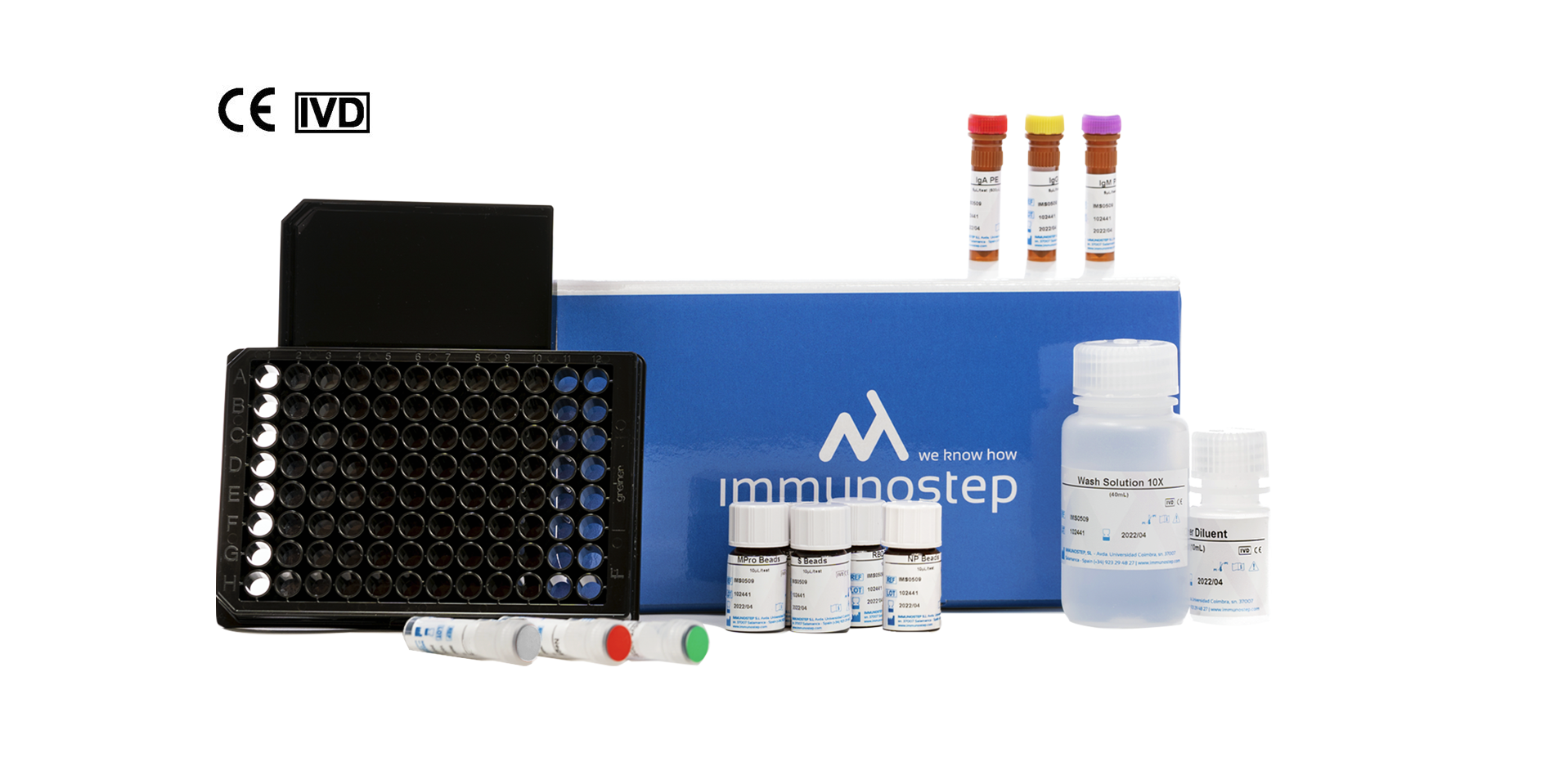
MICROWELL PLATE
12×8 well (12X8) black microtiter with lid. For protocol with plate.
CONTROL SOLUTIONS
- 0.16 ml IgG positive control. Contains CMIT/MIT 3:1 as preservative. Ready to use.
- 0,16 ml IgG negative control. Contains CMIT/MIT 3:1 as preservative. Ready to use.
CALIBRATOR
- 80 μl calibrator. Contains CMIT/MIT 3:1 as preservative. Ready to use.
BEADS
Magnetic polystyrene microspheres with a diameter (µm) 5.5 ±0.2 (CV<5%), coated with the following SARS-CoV-2 antigens:
- RBD – bead population no. 1.
- S – bead population no. 2.
- N – bead population no. 3.
- Mpro – bead population no. 4.
WASH BUFFER SOLUTION
40 ml wash buffer (10X). PBS 10% BSA, pH 7.4 – 10X. Contains 10% albumin in 10mM sodium phosphate, 150mM NaCl, pH 7.4, contains KATHON™ anti-microbial agent.
ANTIBODIES
- 500 µl FITC-conjugated anti-human IgG antibody (5 µl/test).
- 500 µl PE-conjugated anti-human IgA antibody (5 µl/test).
- 500 µl of PE-conjugated anti-human IgM antibody – Cyanine 7 – (5 µl/test).
SAMPLE DILUENT BUFFER
10 ml of sample diluent buffer (1X – Ready to use).
Buffer that minimises non-specific binding, cross-reactivity and matrix interference, with blue dye. Contains CMIT/MIT 3:1 as preservative.
Qualitative and Quantitative Assay
Immunogenicity results are reported as an international standard unit. IU/mL for neutralising antibodies (RBD) and BAU/mL for S, N and Mpro Proteins.
Single reagents available

RBD coated beads
IMS0510
Single Receptor-binding domain of S-glycoprotein (RBD) coated beads.

Spike coated beads
IMS0511
Single stable trimer of the spicule glycoprotein (S) coated beads
Mpro coated beads
IMS0513
Single Main virus protease or 3C-typeprotese (3CLpro,Mpro) coated beads.

Sample Diluent Buffer
IMS0514
Contains < 0.0014 % [w/w] reaction mass of CMIT/MIT (3:1).

Wash Buffer Solution
IMS0515
Contains 10 % albumin in 10mM sodium phosphate, 150 mM NaCl, pH 7.4, contains KATHON anti-microbial agent.
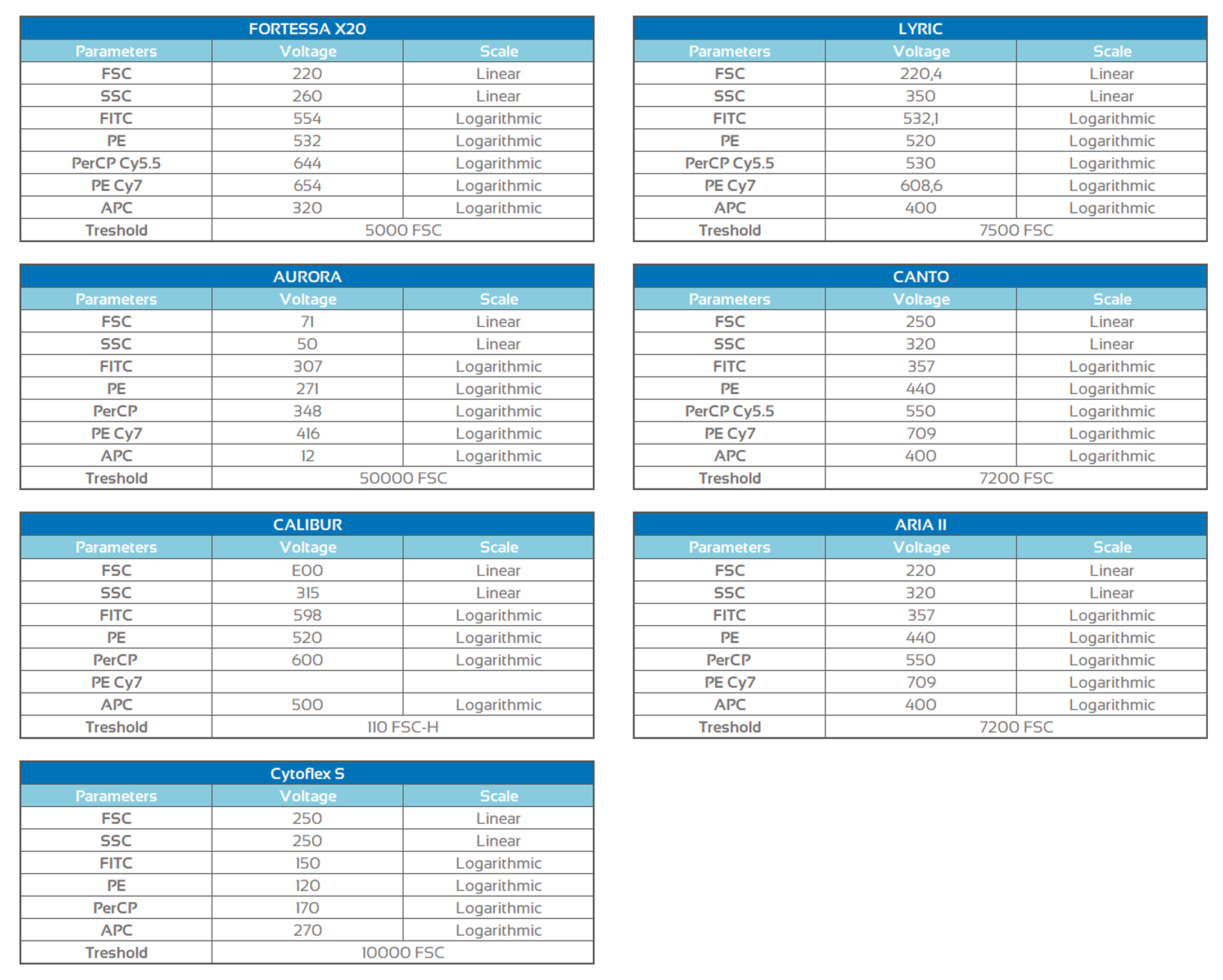
Assay performance
Assay procedure
Qualitative and quantitative analysis of the inmune response
Different serology tests detect antibodies to different parts of the virus. After vaccination, the antibodies will only be formed to one part of the virus (the Spike protein). To detect if vaccination stimulated an antibody response, a test specifically designed for the antibodies of interest is needed.
This test presents a great opportunity because it gives so much information about the immune response based on the detection of 3 types of immunoglobulins and 4 specific SARS-CoV-2 antigens simultaneously, allowing us to differentiate the immune response for clinical infection and vaccination.
FAQS
If you fill the form above indicating your interested kit, one of our specialists will contact you with the exact details.
You can also write at order@immunostep.com with the corresponding reference number you can see in the table.
Or you can contact us by phone at+34 923 29 48 27 (Spain).
CE/IVD kits are only suitable for clinical use in European Union and countries with EU conformity recognition agreements. Manufactured in Spain by Immunostep under CSIC Patent Licence.
The SARS-CoV-2 Multiplex assay has been calibrated against the WHO First International Standard for Anti-SARS-CoV-2 Immunoglobulin (Human), NIBSC Code 20/136.
In this sense, it is possible to quantitatively report the concentration of IgG immunoglobulins in International Units (IU / ml) against RBD or in United Antibody Units (BAU / ml) against S, N and Mpro. For this reason, the kit includes a calibrator or standard of known concentration with which to make a calibration line through serial dilutions to each of the viral antigens used in the test (RBD, S, N, Mpro) in which to interpolate the fluorescence values resulting from the assay of the samples, thus obtaining the concentration in IU / ml or BAU / ml corresponding to each sample.
These tests are for professional use only. If you are interested in acquiring these kits, please, contact us by email at info@immunostep.com, and we will contact you shortly.
If you are a particular and you want to acquire this test, please contact us in order to recieve a personalized proposal of our partners near you.
Our products are available through our extensive distribution network all over the world. Please contact us and we will give you all the information shortly.

Project subsidized by the Ministry of Industry, Trade and Tourism, as part of the aid program for the manufacture of health material for the COVID-19 crisis (2020) with the objective of creating a production line of SARS-CoV-2 Multi-antigen serological tests for Flow Cytometry.