- Products
- Oncohematology
- Antibodies
- Kits
- CAR T-cell
- Euroflow
- Single reagents
- Request info
- Resources and support
- Immunology
- Antibodies
- Single reagents
- Cross match determination (FCXM)
- FcεR1
- Ig subclasses
- Single reagents
- Kits
- TiMas, assessment of tissue macrophages
- Request info
- Resources and support
- Antibodies
- Exosomes
- Accesory reagents
- Software
- Oncohematology
- Services
- Peptide Production
- Design
- Modification
- Protein Services
- Expression and purification
- Freeze drying
- Monoclonal And Polyclonal Antibody Development
- Monoclonal
- Policlonal
- Specialized antibody services
- OEM/Bulk production
- Purification
- Conjugation
- Custom Exosome Services
- Isolation and purification
- Characterization
- Peptide Production
- Shop
- Support
- About Us
- Contact

Anti-SARS-Cov-2 Mpro ELISA Test
The first serological assay designed to detect with high specificity and sensitivity recent and past SARS-CoV-2 infection.
Innovative serological test
It detects antibodies from the onset of symptoms, allowing to confirm patients with recent and past infection.
Anti-SARS-CoV-2 Mpro ELISA kit
Anti-SARS-Cov-2 ELISA kit is a highly sensitive and specific test developed by Immunostep under CSIC patent licence, for the precise detection of either IgG or IgA antibodies against the Mpro (3CLpro) Protein of SARS-Cov-2 virus.

Aids in diagnosis & patient surveillance

Validated by the immunology services of important hospitals and laboratories
DESIGNED TO BE USED IN HUMAN SERUM AND PLASMA SAMPLES
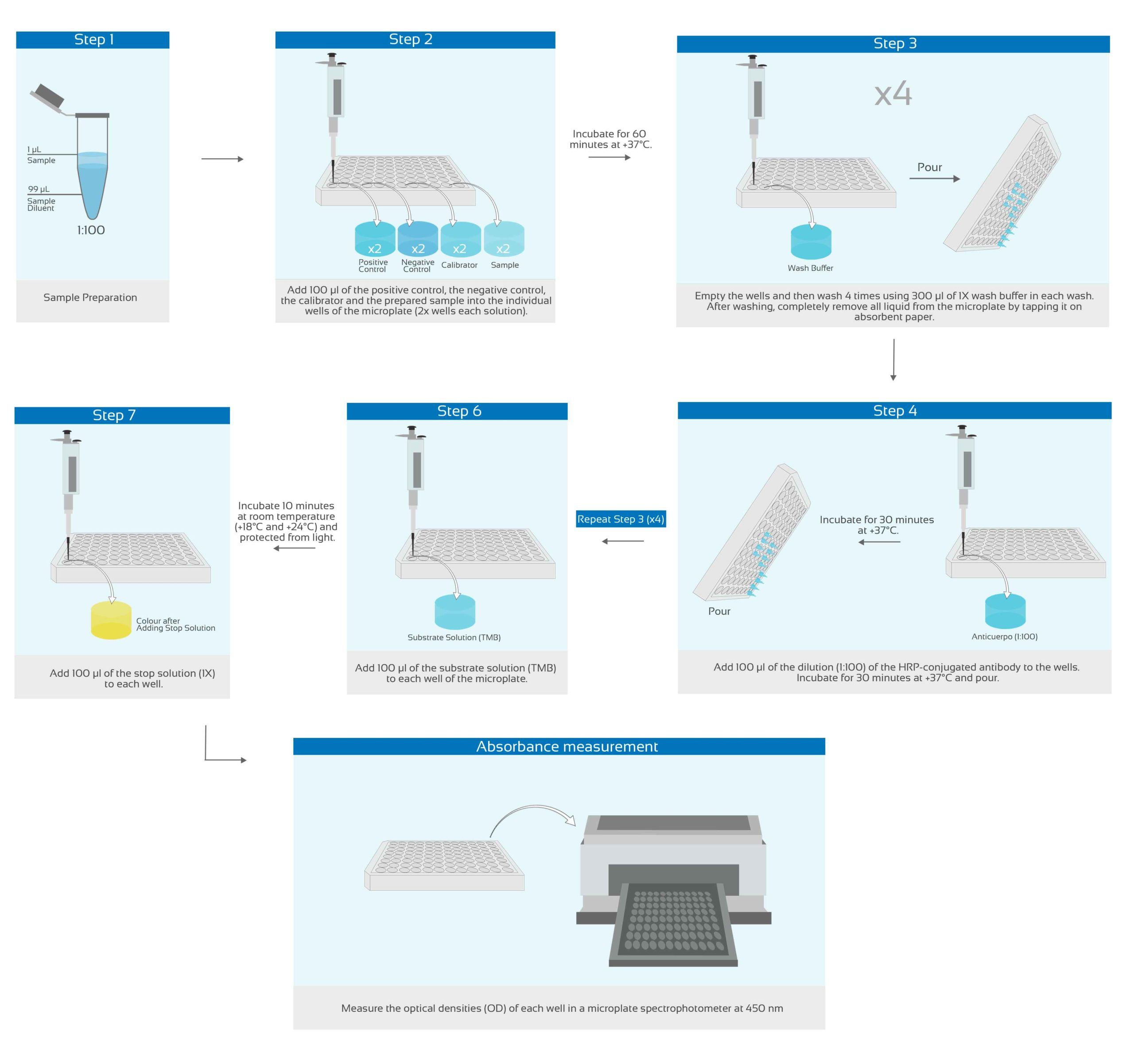
What this kit includes?
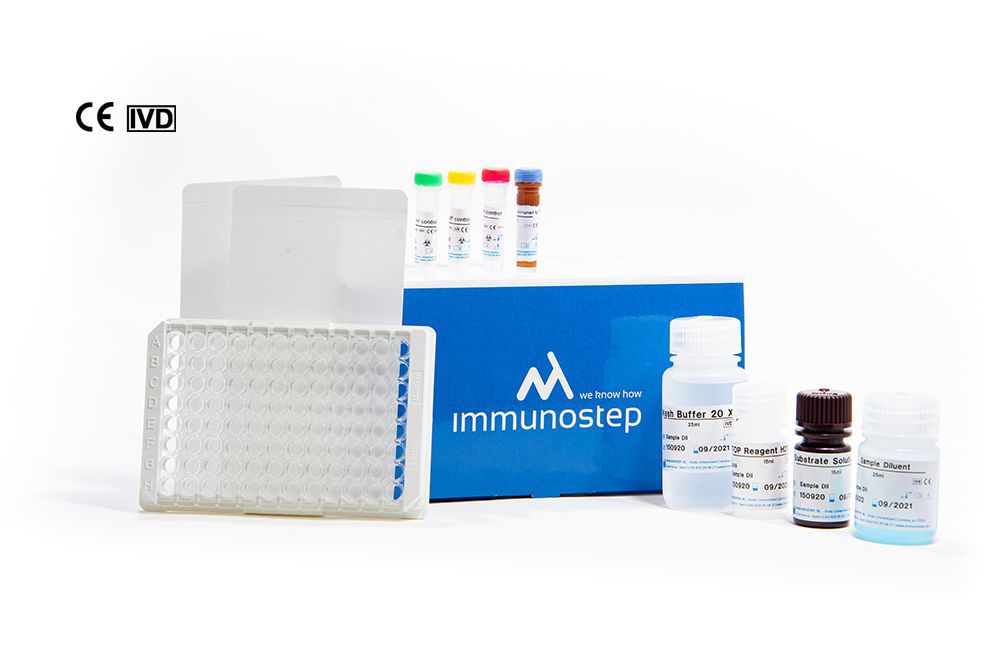
Microwell plate
12×8 wells microtiter strip plate coated with recombinant SARS-CoV-2 antigen in vacuum-sealed pouch. 2 microwell sealing foils.
IgG/IgA control solutions
- 5 ml Positive Control/ Contains CMIT / MIT 3: 1 as a preservative. Ready to use.
- 5 ml cut-off. Contains CMIT / MIT 3: 1 as a preservative. Ready to use.
- 5 ml Negative Control. Contains CMIT / MIT 3: 1 as a preservative. Ready to use.
HRP-Conjugated Antihuman Antibody
120 µl of HRP-conjugated anti-human antibody (100X). Contains CMIT / MIT 3: 1 as a preservative.
Wash buffer
50 ml wash buffer (20X). HEPES, NaCl and detergents. Contains Proclin300 as a preservative (<0.0014%).
Stop Solution
12 ml Stop Solution (1X – Ready to use). 0.5M sulfuric acid (H2SO4)
Tetramethylbenzidine (TMB)
12 ml of tetramethylbenzidine (TMB) chromogenic substrate. (1X – Ready to use)
Antibody and Sample Diluent Buffer
25 ml of Antibody and Sample Diluent Buffer (1X – Ready to use). Buffer that minimizes non-specific binding, cross-reactivity and matrix interference, with blue dye. Contains CMIT / MIT 3: 1 as a preservative.
About Mpro Protein
About the Assay
FAQs
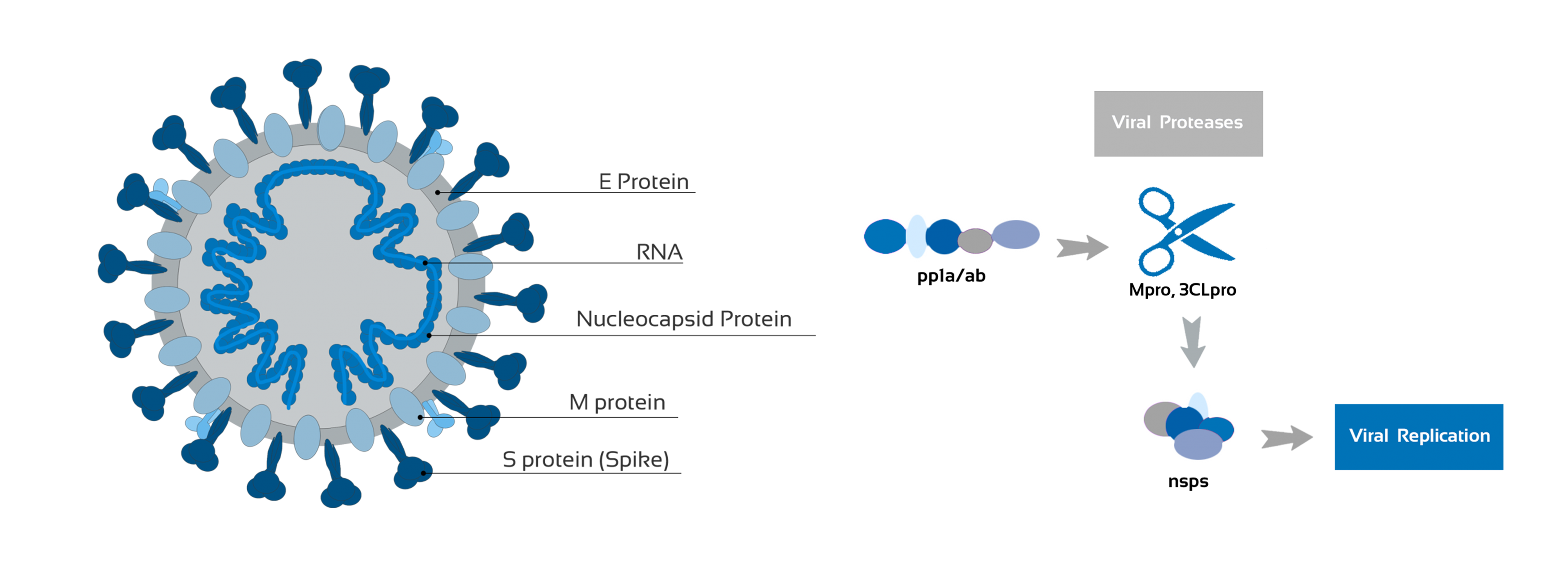
Vital Role In Viral Replication
In an attempt to increase the diagnosis possibilities of COVID-19 patients, this assay was developed for the detection of specific antibodies against one of the 16 non-structural proteins, the main viral protease (Mpro or 3CLpro), which plays a critical role in viral replication.
The Mpro protease plays a vital role in processing the polyproteins that are translated from the viral RNA. Although this protein is not exposed in the viral particle, Mpro is elicited after viral infection. Like other b-coronaviruses, SARS-CoV-2 is a positive-sense RNA virus that expresses multiple proteins as a single polypeptide chain, and Mpro cleaves the polyprotein to release mature proteins for the virus. Inhibitors that can block viral replication are promising potential drug candidates that could be used to treat patients suffering from the COVID-19 infection.
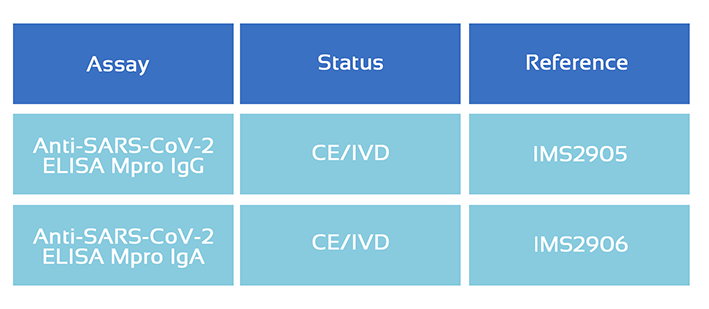
If you fill the form above indicating your interested kit, one of our specialists will contact you with the exact details.
You can also write at order@immunostep.com with the corresponding reference number you can see in the table.
Or you can contact us by phone at+34 923 29 48 27 (Spain).
CE/IVD kits are only suitable for clinical use in European Union and countries with EU conformity recognition agreements. Manufactured in Spain by Immunostep under CSIC Patent Licence.
These tests are for professional use only. If you are interested in acquiring these kits, please, contact us by email at info@immunostep.com, and we will contact you shortly.
If you are a particular and you want to acquire this test, please contact us through the form below in order to recieve a personalized proposal of our partners near you.
Our products are available through our extensive distribution network all over the world. Please contact us and we will give you all the information shortly.