- Products
- Oncohematology
- Antibodies
- Kits
- CAR T-cell
- Euroflow
- Single reagents
- Request info
- Resources and support
- Immunology
- Antibodies
- Single reagents
- Cross match determination (FCXM)
- FcεR1
- Ig subclasses
- Single reagents
- Kits
- TiMas, assessment of tissue macrophages
- Request info
- Resources and support
- Antibodies
- Exosomes
- Accesory reagents
- Software
- Oncohematology
- Services
- Peptide Production
- Design
- Modification
- Protein Services
- Expression and purification
- Freeze drying
- Monoclonal And Polyclonal Antibody Development
- Monoclonal
- Policlonal
- Specialized antibody services
- OEM/Bulk production
- Purification
- Conjugation
- Custom Exosome Services
- Isolation and purification
- Characterization
- Peptide Production
- Shop
- Support
- About Us
- Contact
CAR T-cells:
High resolution recognition and discrimination with a single reagent
With the increasing importance of CAR T-cell therapy in cancer treatment, ensuring the successful modification and monitoring of these engineered T-cells is crucial.
Our Flow Cytometry Reagent simplifies this process, allowing specialists to confirm both key challenges with an easy 1 hour protocol.
Experience the power of precision and efficiency recognising and discriminating CAR T-cells from the rest with high resolution.
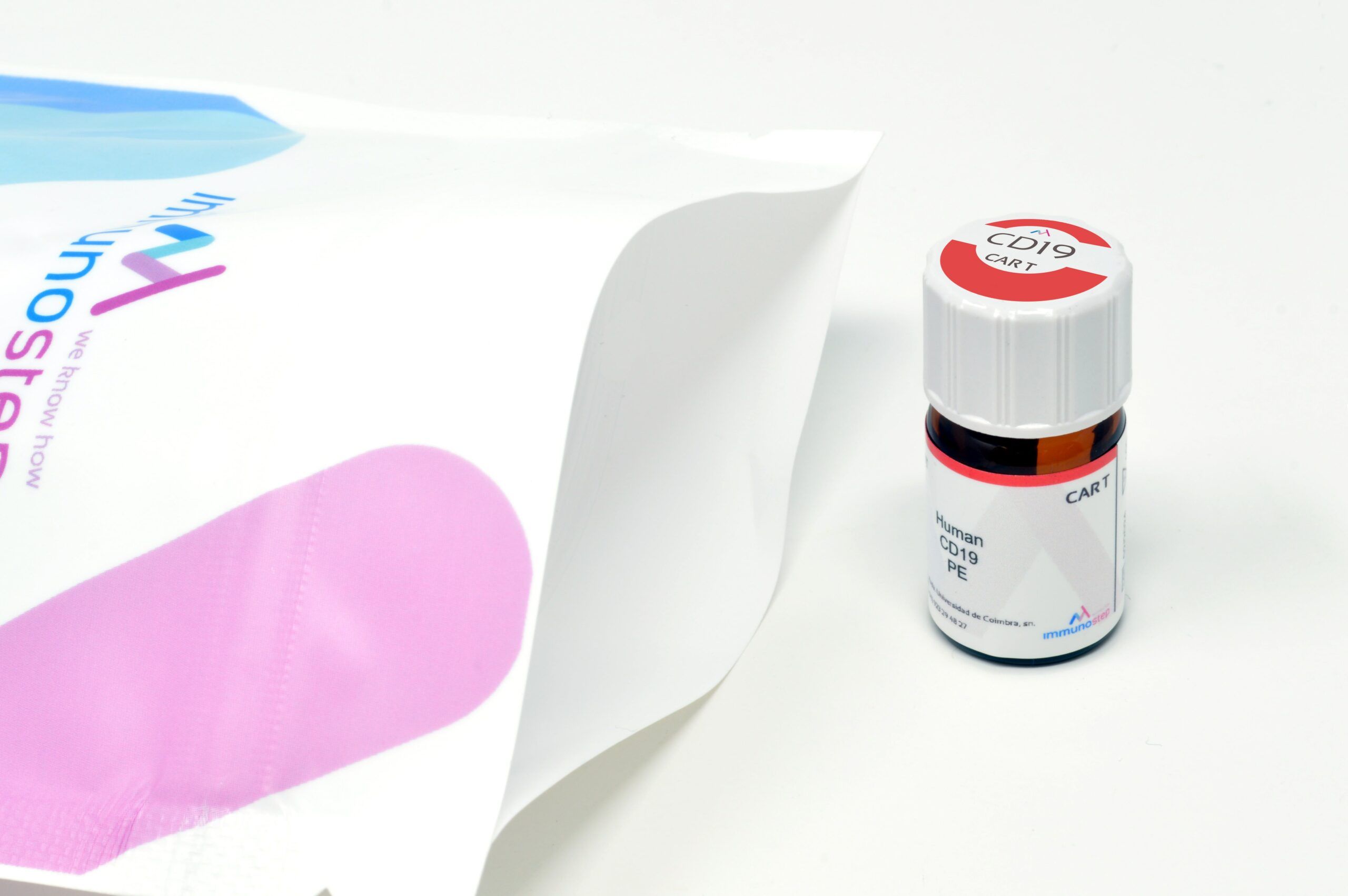
PRODUCT REFERENCES
| Product name | Reference | Description | |
|---|---|---|---|
CD19 CAR-T | 19-0212-25T | Human PE-CD19 | RUO | 25 test | Go to shop |
CD19 CAR-T | 19-0212-100T | Human PE-CD19 | RUO | 100 test | Go to shop |
CD19 CAR-T | 19-0213-25T | Human FITC-CD19 | RUO | 25 test | Go to shop |
CD19 CAR-T | 19-0213-100T | Human FITC-CD19 | RUO | 100 test | Go to shop |
BCMA CAR-T | BCMA-0312-25T | Human PE-BCMA | RUO | 25 test | Go to shop |
BCMA CAR-T | BCMA-0312-100T | Human PE-BCMA | RUO | 100 test | GO TO SHOP |
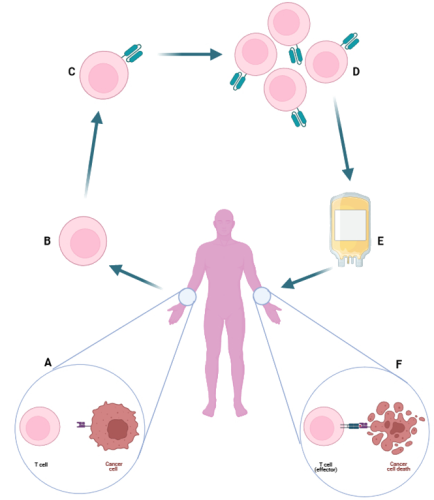
CAR T-cells therapy is based on the extraction of patient T-cells, the introduction of a Chimeric Antigen Receptor (CAR) specific to a protein present on cancer cells (e.g. CD19, BCMA) and the consequently reinfusion of these modified cells, now called CAR T-cells, into the patient to recognise the target cells and eliminate them.
This therapy has gained relevance due to its efficacy, and detecting CAR T-cells tools has become a critical need, both to check that they have been correctly modified, and to monitor them after the reinfusion, since they have a limited life span.
Figure 1: Basic scheme of CAR T-Cell therapy. (A) Own T-Cell does not recognize cancer cell. (B) T-Cell extraction. (C) Modification of T-Cell with CAR. (D) CAR T-Cell cultivation and harvesting. (E) Harvested CAR T-Cells are reinfused into the patient. (F) CAR T-Cell recognizes and eliminates cancer cell.
A differential solution
Identify CAR T-cells from other populations with High Resolution, even with difficult samples

Unique reagent needed.
Super simple 1 hour protocol.
Fluorochrome compatible with
any Flow Cytometer (R-PE)

Compatible with panels of
more than 30 antibodies
Limitations of Current Reagents
There are some reagents for identifying and quantifying CAR T cells, based on fluorescent labeled (PE or FITC) recombinant CD19 or BCMA. However, current reagents have limitations in separating the desired populations with sensitivity, making it more difficult for the analysts to provide an accurate and reliable result.
Those reagents that overcome this problem have the disadvantage of involving more complex and time-consuming protocols, using an amplification reagent in addition to the protein. It is therefore necessary to introduce new simple, straightforward and easy to use reagents that are able to clearly discriminate the CAR T-Cells from the rest of populations for a more reliable analysis.
Figure 2: FACS (Fluorescent-Activated Cell Sorting) protocol. Immunostep vs competitors. Other competitors require more steps and reagents (competitor 1) or longer protocols (competitor 2). Immunostep combines the simplicity with the speed, providing analysts with an easier protocol.
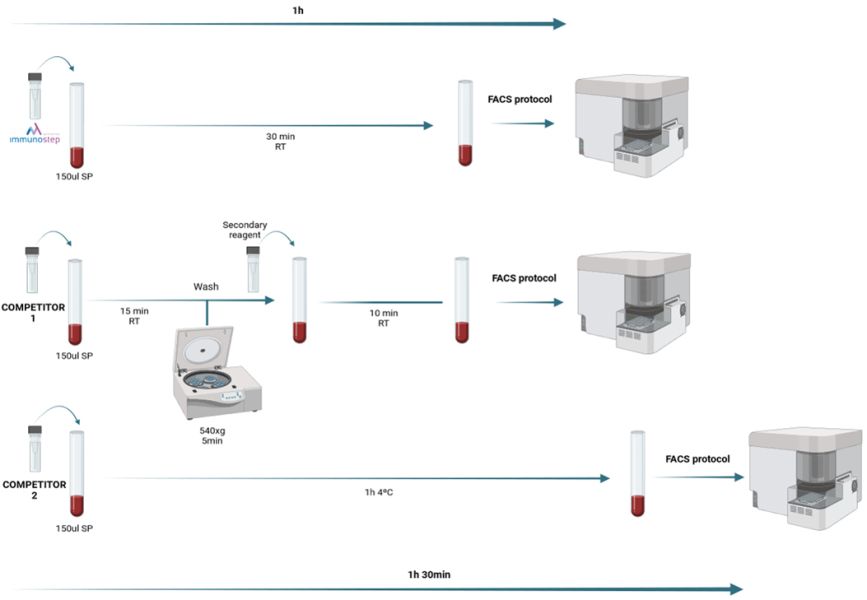
The reagent is supplied in aqueous solution for direct cell labelling with 5 μl of it. This, with the use of an antibody panel to discriminate T-Cells, is sufficient to separate those which have CAR in just a 30 minutes incubation at room temperature (RT) spending around 1 hour of complete protocol, whereas other protocols spend same time just to label the specific population (see figure 2).
Other commercially reagents, such as CD19 or BCMA CAR Detection Reagent are based on an indirect staining, with a biotinylated protein that must be revelated by a fluorescent secondary reagent. This strategy has good results, but a high cost and a more complex protocol.
Detection of CAR T-Cells
To meet these needs, we have developed a single reagent for direct and specific recognition of CAR T-Cells with high resolution. This reagent allows the specialists to confirm the successful modification of T-Cells in a quality control prior infusion into the patient and to monitor them after this step.
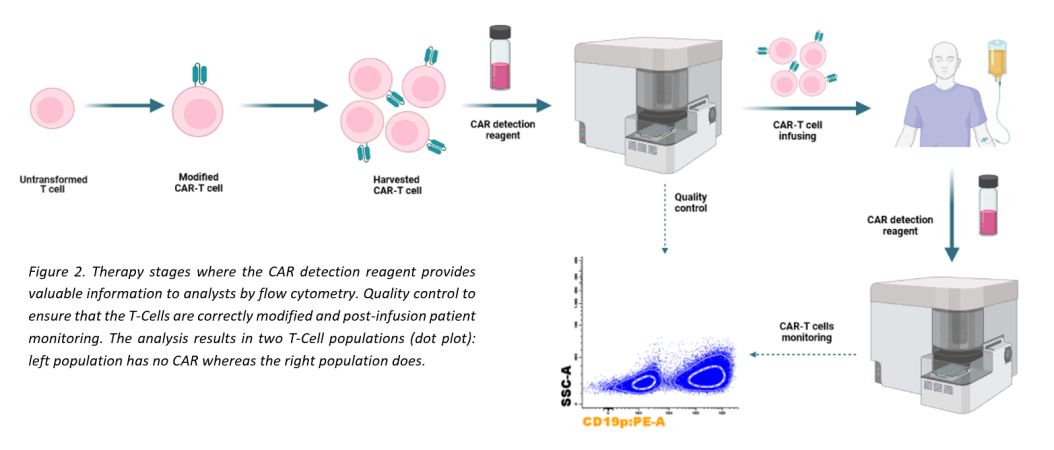
TECHNOLOGY
Recombinant Fc-Tag CD19 protein (CD19p) and BCMA protein (BCMAp) has been labeled, by a proprietary process, with R-Phycoerythrin fluorochrome (R-PE or PE) or FITC, obtaining a conjugate that is able to be recognized by CAR T-Cells and separates them efficiently without any secondary reagent.
Other manufacturers have similar products based on this fluorochrome-protein complex but have limitations in the separation due to bioconjugation techniques and/or the fluorochrome used.
BrightStepTM: our proprietary labeling technology
This cutting-edge technology is set to redefine CAR-T cell detection. With BrightStep, we’ve improved the art of labeling, ensuring the maintenance of conformation and natural modifications of the protein. This means unparalleled accuracy without compromising the integrity of the sample.
Verified by rigorous flow cytometry testing, Brightstep offers higher performance in specificity and sensitivity. You can trust in its reliability to deliver precise results every time.
Furthermore, with batch-to-batch consistency and reproducibility, you can rely on BrightStep for ensuring reliable outcomes for your research and diagnostics needs.
Experience the future of CAR-T cell detection with BrightStep – where innovation meets accuracy.
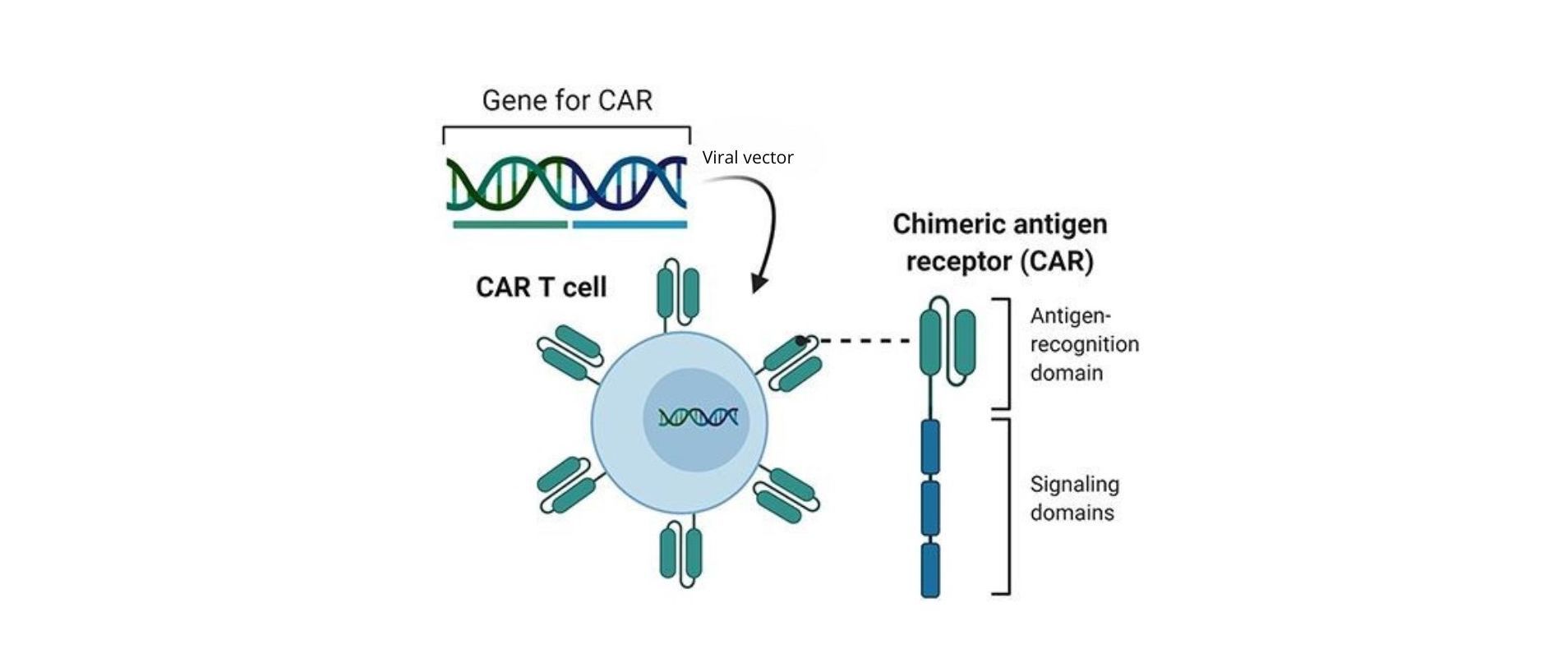
Figure 4: Representation of the T-Cell modification to CAR T-Cell and the CAR structure. The T-Cell is transfected with a viral vector containing the information to express the CAR. This is composed of an extracellular domain that specifically recognizes the antigen, designed from an antibody, and other transmembrane and intracellular domains with signaling and co-stimulatory functions that increase the efficiency of the T-Cell recognition and activation.
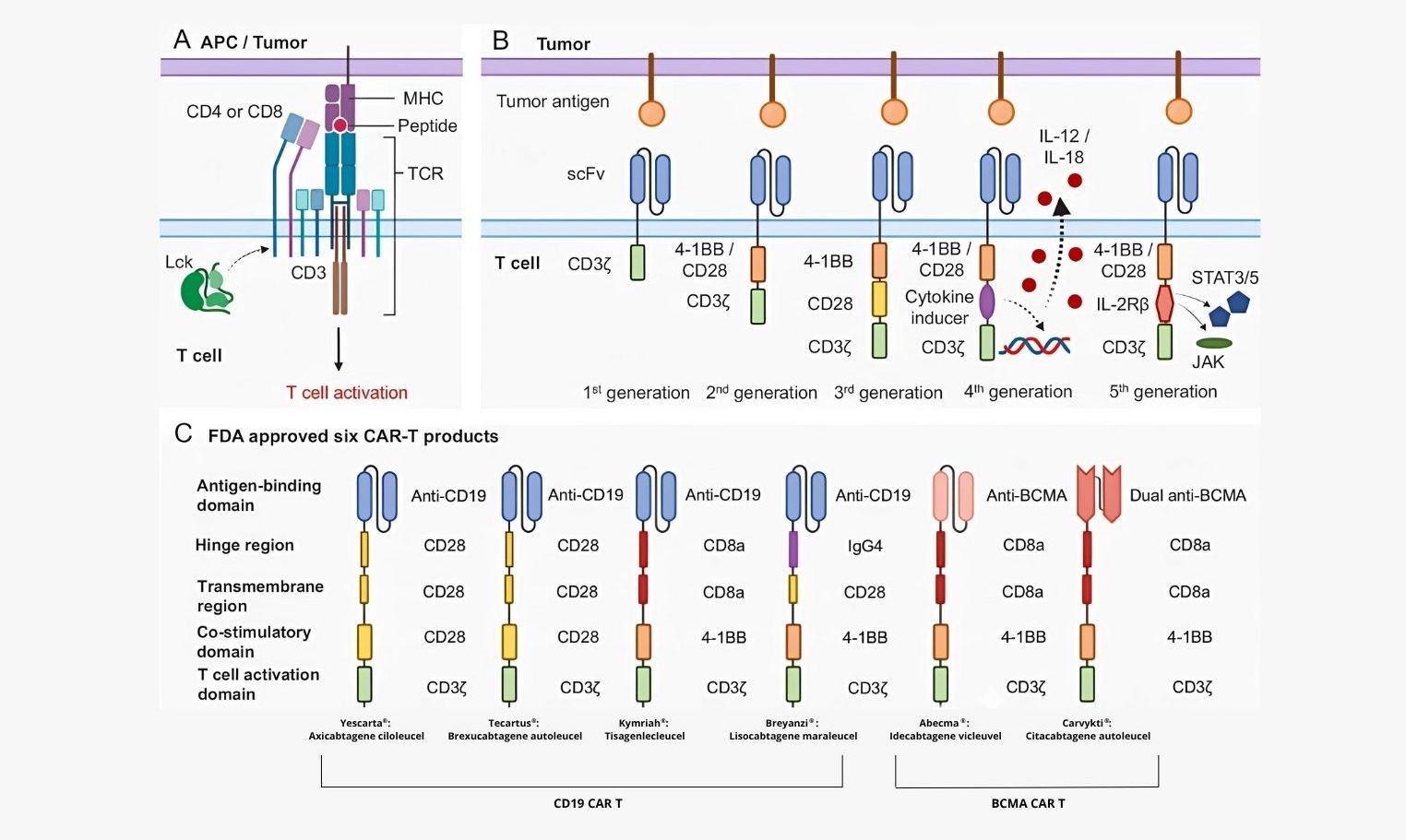
Figure 5: Chimeric antigen receptor (CAR) construct. A When a T cell encounters antigen-presenting cells or tumor cells, T cell receptor (TCR) and CD3 complex, together with CD4 or CD8, will recognize MHC complex-presented peptides, then trigger TCR signaling cascade, leading to T cell activation. B The first-generation CAR majorly involves two parts: scFv at the extracellular part and CD3ζ chain at the intracellular part. ScFv domain can recognize tumor surface antigens, and then the CD3ζ chain will directly activate T cells. The second-generation CAR incorporated a co-stimulation domain, either 4-1BB or CD28 signaling domain. The third-generation CAR has both 4-1BB and CD28 signaling domains. C Differences of antigen-binding domain, hinge region, transmembrane region, co-stimulatory domain, and T cell activation domain of six FDA-approved CAR-T products. Those products are used to treat B-cell lymphoma, B-cell leukemia, and multiple myeloma. APC antigen-presenting cell, MHC major histocompatibility complex, TCR-T cell receptor, scFv single-chain variable fragment. (Source: Nature)
COMPARISON BETWEEN SOLUTIONS
The fundament of flow cytometry is binding the reagent to a specific population and discriminating it by the reagent fluorescence. The reagent targets the CAR in the engineered T cell, so the separation depends on the level of expression of the CAR and the discriminatory ability of the reagent. The reagent identifies the specific population by his fluorescence, but it is not so important the intensity of that fluorescence, measured as MFI (Median Fluorescence Intensity), but the ability to discriminate between the positive population (CAR T-Cells) and the negative populations (rest of the cells).
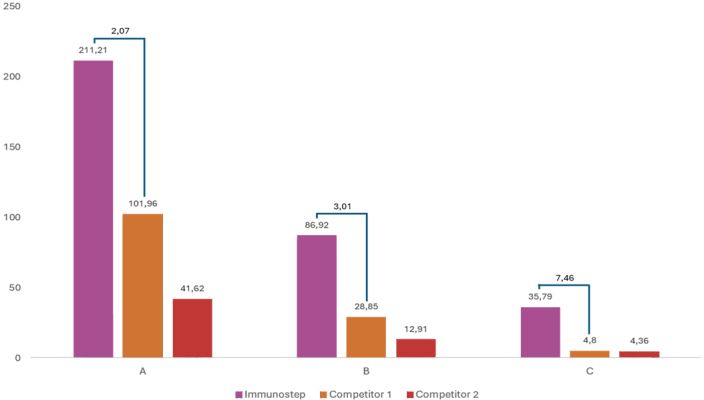
Figure 6: A bar graph representing the ratio between Immunostep and other two competitors. In sample C, the most difficult discrimination (academic CAR T-Cells), Immunostep shows highest difference, providing reliable result.
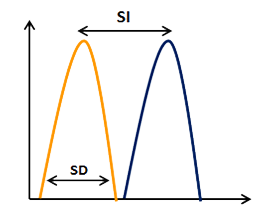
It means that it is necessary the greatest difference between both populations MFI; this value is known as Stain Index (SI), the greater SI the better discrimination. A highly fluorescent reagent is needed when the amount of target molecule is low but sometimes also increases the negative MFI, getting worse the SI. Best reagents have high SI with a moderate or high fluorescence to identify CAR T-Cells, even with low CAR expression.
Figure 7: Stain Index, value to estimate the level of discrimination between positive and negative populations.
STAIN INDEX = (Median of Positive – Median of Negative) / (SD of Negative * 2).
Frequently Asked Questions (FAQs)
The PE CD19 CAR Detection Reagent has a stability of one (1) year in refrigerated conditions (4 – 8 ºC). Avoid freezing and constant temperature changes.
100-200 µl of peripheral blood can be labeled with 5 µl (1 test) of PE CD19 CAR Detection Reagent. Depending of the sample, it may need to be titrated.
R-Phycoerythrin (R-PE or PE) can be excited at 488 nm (Blue Laser). Most of flow cytometers have this excitation laser, so PE CD19 CAR Detection Reagent can be detected by any ot them.
Yes, it has been tested in panels of up to 33 reagents and still works. If the panel has many reagents, it may need to be titrated for better results.