- Products
- Oncohematology
- Antibodies
- Kits
- CAR T-cell
- Euroflow
- Single reagents
- Request info
- Resources and support
- Immunology
- Antibodies
- Single reagents
- Cross match determination (FCXM)
- FcεR1
- Ig subclasses
- Single reagents
- Kits
- TiMas, assessment of tissue macrophages
- Request info
- Resources and support
- Antibodies
- Exosomes
- Accesory reagents
- Software
- Oncohematology
- Services
- Peptide Production
- Design
- Modification
- Protein Services
- Expression and purification
- Freeze drying
- Monoclonal And Polyclonal Antibody Development
- Monoclonal
- Policlonal
- Specialized antibody services
- OEM/Bulk production
- Purification
- Conjugation
- Custom Exosome Services
- Isolation and purification
- Characterization
- Peptide Production
- Shop
- Support
- About Us
- Contact
Peptide Production
A peptide consists of a compound, either natural or synthetic, that contains two or more amino acids linked by a carboxyl group of one of the amino acids and the amino group of another. Peptide molecules are structurally the same as proteins but are generally smaller. There are many peptides that function in diverse ways, for example as hormones, antibiotics and other compounds which participate in many of the metabolic activities of living organisms.
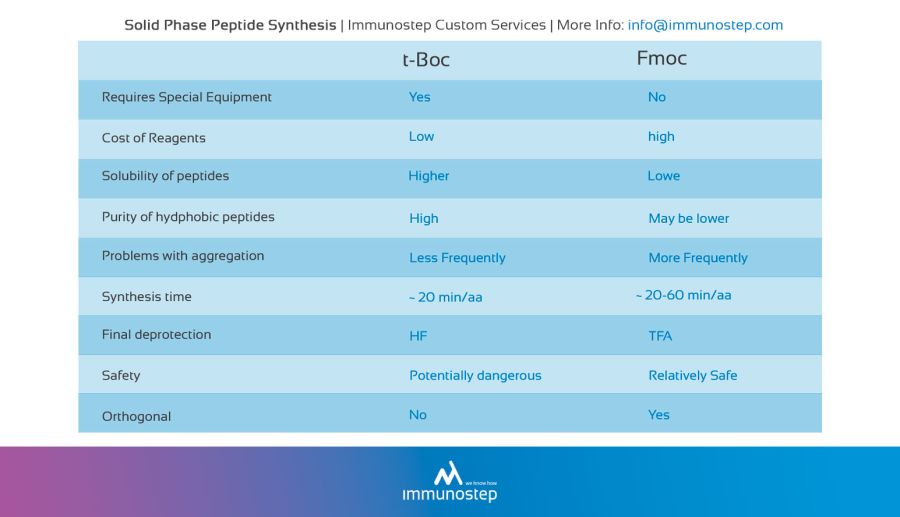
Solid Phase Peptide Synthesis.
This peptide synthesis method is based on the incorporation of N-α-amino acids into the sequence of any required peptide anchored to a solid matrix which acts as a support. In the peptide synthesis process, soluble reagents are generally washed away at the end of the addition (of each amino acid). Once the desired peptide is obtained, it is freed from the polymeric support.
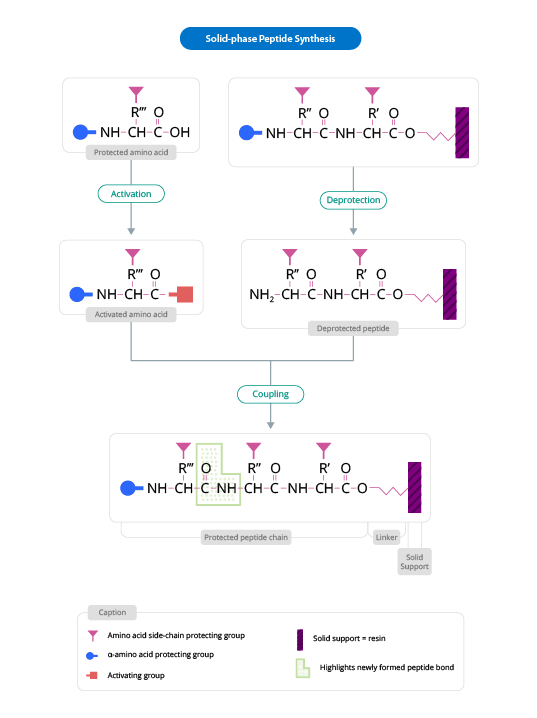
Solid support
The solid support is a synthetic polymer that becomes attached to groups of reagents with -OH.
These groups are designed so that they can easily react with the carboxyl group of an N-α-amino acid, protected at its carboxyl terminus; it is thus covalently attached to the polymer.
The protection of the amine group (X) may be removed, and a second N-α-amino acid will become attached to the anchored amino acid. The steps described are repeated until the desired sequence is obtained. When the synthesis has finished, a reagent releases the carboxyl terminus from its attachment to the matrix, leaving the required peptide in solution
Since 2001, we have designed and synthesized a multitude of peptides that have been used by our customers in virtually all of their areas of work, most especially in research.
We are looking forward to hearing about your needs
Peptide Design
Peptide synthesis can be simple, but a series of factors must be respected before commencing the synthesis. The sequence, composition of amino acids and length of peptides can influence the correct assembly of amino acids, and also later purification. These factors will affect the solubility of the final product. When designing peptides, the following points should be considered so that our customers receive the ideal product to suit their needs:
This affects not only the ease of assembly of amino acids, but also solubility, stability and the later purification process.
Most peptides of interest, such as those used as antigens, proceed from N-term ends, C-term or from the interior of a sequence of native proteins.Thus these peptides are not always ideal.
Some can be insoluble or unstable, others can cause purification problems, whilst, in other cases, folding may not be similar to that of native proteins.
Thanks to the experience we have acquired over the years, at Immunostep we can locate those problematic amino acids and modify them by improving the qualities of synthesised peptides.
Several different modifications can be made during production according to our customers’ needs, for example covering N-term or C-term regions, as required, carrying out chemical modifications, inserting spacers, or replacing “problematic” amino acids.
One of the most critical factors in synthesis and purification is excessive length. For instance, at Immunostep we recommend never exceeding 10-15 amino acids for peptides used as antigens, since they will be ideal as they maintain the structure of the linear epitope in native proteins.
Hydrophobicity affects peptide solubility considerably; in this sense, our experience allows us to increase peptide solubility by adapting the frequency of the different amino acids depending on their hydrophilic and/or hydrophobic characteristics.
High incidence peptides such as tryptophan, leucine, valine, methionine, phenylalanine, isoleucine may not dissolve in aqueous solutions, and this allows us to maintain the ratio of these hydrophobic amino acids below 50% while securing the existence of at least one charged amino acid per five residues.
At physiological pH, arginine, glutamine, aspartic acid and lysine are charged on their side chains. One simple change or the elimination of some polar residues at the N-term or C-term ends may influence solubility either positively or negatively.
During the process of peptide synthesis, the formation of β sheets may affect the solvation and ordered synthesis of the peptide, causing losses in its different zones.
We can reduce the number of these deletions and the appearance of unwanted products in the synthesis by recommending the elimination of zones in which there are multiple or adjacent residues of valine, isoleucine, tyrosine, phenylalanine, tryptophan, leucine, glutamine or threonine. In most cases, conservative changes, as well as other measures applied during synthesis can improve chances of success with regard to purity and quality.
Measures such as the insertion of glycine or proline, every three residues, or the replacement of glutamine for asparagine, or threonine for serine.
Peptide Modification
We often find that our customers require diverse modifications to the peptides which have already been developed. Such modifications are common in different experiments and require the experience of a group that is accustomed to dealing with them.
-
D- amino acids
-
C-term modifications: Amidation, lys(Biotin), Lys (FAM)
-
C-term modifications: Amidation, lys(Biotin), Lys (FAM)
-
N-term modifications: Acetylation, Myristic Acid, Palmitic acid, Formic acid, Biotin.
-
N-term Fluorescent Labelling: FAM, FITC, TAMRA
-
Lys Side Chain Modifications: Lys (AC), Lys (Biotin)
-
Phosphorylation. Phosphorylation-Ser, Phosphorylation-Thr, Phosphorylation-Tyr
-
Cyclization. First Disulfide Bridge, Second Disulfide Bridge, head-to-tail cyclization
-
Conjugation. KLH BSA
-
Multiple Antigen Peptide (MAP) 4 branches, 8 branches