- Products
- Oncohematology
- Antibodies
- Kits
- CAR T-cell
- Euroflow
- Single reagents
- Request info
- Resources and support
- Immunology
- Antibodies
- Single reagents
- Cross match determination (FCXM)
- FcεR1
- Ig subclasses
- Single reagents
- Kits
- TiMas, assessment of tissue macrophages
- Request info
- Resources and support
- Antibodies
- Exosomes
- Accesory reagents
- Software
- Oncohematology
- Services
- Peptide Production
- Design
- Modification
- Protein Services
- Expression and purification
- Freeze drying
- Monoclonal And Polyclonal Antibody Development
- Monoclonal
- Policlonal
- Specialized antibody services
- OEM/Bulk production
- Purification
- Conjugation
- Custom Exosome Services
- Isolation and purification
- Characterization
- Peptide Production
- Shop
- Support
- About Us
- Contact
Fluorescent dextrans
An alternative to classical fluorescent labelling methods for flow cytometry.
Better signal and greater versatility.
PRODUCT REFERENCES
| Product name | Reference | Description | |
|---|---|---|---|
Fluorescent Streptavidin Dextrans-25T | DXPESTV-25T | PE Fluorescent Streptavidin Dextrans | RUO | 25 test | GO TO SHOP |
Fluorescent Streptavidin Dextrans-100T | DXPESTV-100T | PE Fluorescent Streptavidin Dextrans | RUO | 100 test | GO TO SHOP |
Dextrans, polysaccharides extracted naturally from bacteria, are used in medicine for numerous applications as carriers for drugs or vaccines, but can also be used to incorporate other molecules of interest.
Such is the case of fluorescent dextrans, where fluorochromes, whether synthetic (FITC) or protein (PE or APC), and streptavidin, a protein with a high affinity for biotin and with four available binding sites, are coupled.
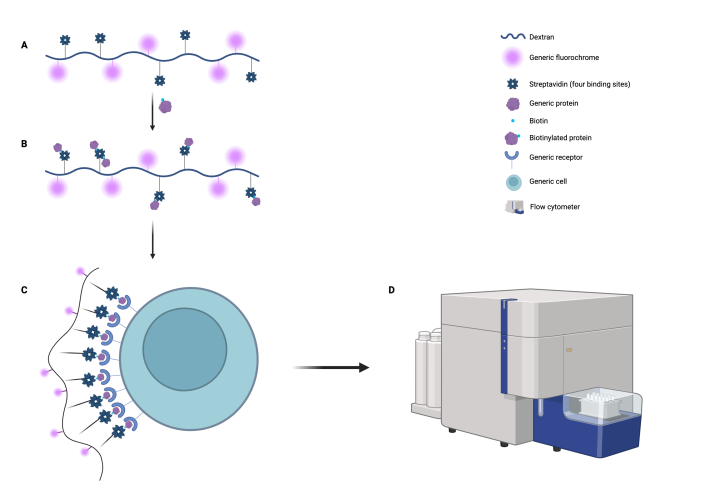
This dextran-fluorochrome-streptavidin complex (Fig. 1A) allows any biotinylated molecule to be bound via the biotin-streptavidin system (Fig. 1B).
This incorporated molecule, antigen, can be used to bind the whole complex to a target cell, or other established system, (Fig. 1C) and be detected by flow cytometry by means of the fluorochrome (Fig. 1D).
Figure 1: Dextran signalling system
New pathway
This system is an alternative to conventional conjugations between proteins of interest (antigens) and fluorochromes; these conjugations are direct in the sense that a link is created between the two molecules. Dextran offers certain advantages over the conventional method:
-
-
Efficient antigen recognition. The antigen is not directly conjugated to the fluorochrome, which often hinders recognition due to steric hindrance, but binds to the streptavidin and is distant and recognisable due to the space created between molecules throughout the dextran. This greatly enhances antigen-receptor binding. It is preferable that the antigen is monobiotinylated so as not to compromise the recognition zone.
-
Optimised antigen presentation. It is more efficient because it is a multimer; there are numerous antigen molecules on the dextran, making it more likely that one or more will be recognised and the binding more stable.
-
Signal amplification. Able to discriminate positive from negative population with very low amounts, as the presentation and recognition is optimised and not too high a titre is needed.
-
Low non-specific labelling. By not having to add high amounts in order to separate the specific population, non-specific labelling is significantly reduced, grouped and identifiable.
-
Detection of minority populations. All of the above qualities contribute to being able to discriminate very small populations (below 0.5%) with high resolution.
-
Fast and efficient conjugation. Being based on a biotin-streptavidin system, conjugation takes only 30 minutes; it is necessary to test different amounts to establish the best ratio of biotinylated protein to dextran, but no purification steps are required, as the best ratio offers maximum efficiency without losses.
-
Antigen-receptor binding
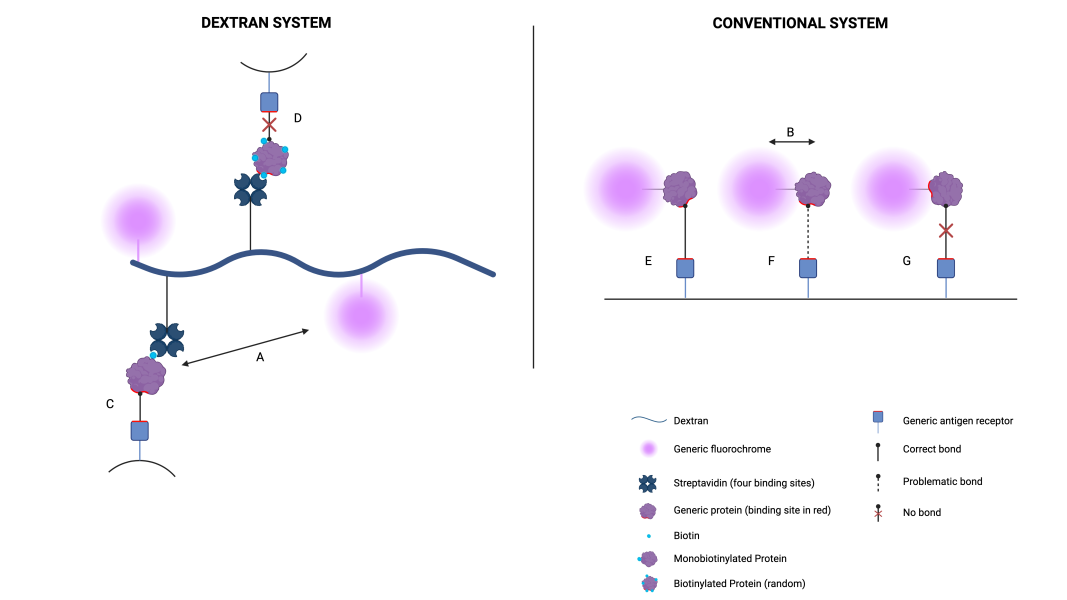
Figure 2: Antigen-receptor binding comparing dextran conjugation with conventional conjugation.
A-B) We note the difference in spacing between the fluorochrome and the protein; the spacing in the dextran (A) is larger than in a conventional conjugation (B), which favours binding, as can be seen in the comparison of bindings C and F. The binding site in F is partially impeded by the spacing of the fluorochrome.
C-D) We compare the binding of a monobiotinylated protein (C) to a random normal biotinylated one (D). It is observed that random conjugation compromises the recognition of the protein, as the binding site may be conjugated to biotin and bind to streptavidin or the protein may become spatially unrecognisable. Monobiotinylation is targeted and the binding site is secured.
E-F-G) In a conventional conjugation, three situations can occur with respect to binding: correct binding (E), problematic binding (F) and no binding (G). This occurs because the conjugation is random and the binding site can be more or less accessible; this does not occur in dextran (as seen in C).
Cell labelling
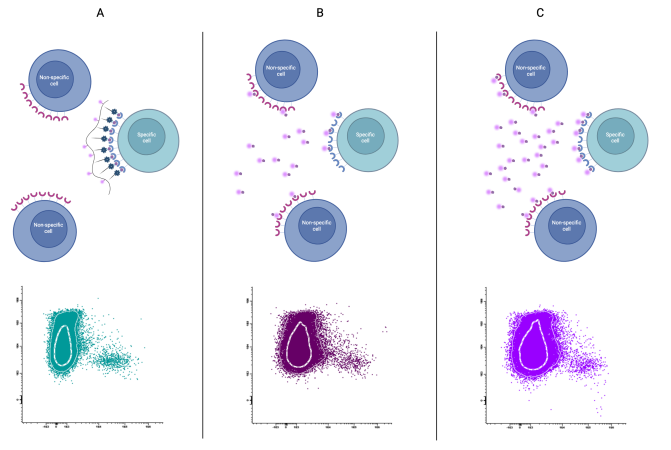
Figure 3: We compared the fluorescent dextran cell labelling system (A) with the conventional system (B and C).
A) We note that it is not necessary to add a large amount of dextran to correctly label the specific population, since it is an efficient binding. This means that we avoid adding too much reagent that causes non-specific labelling and, therefore, we will achieve an optimal separation.
B) With a low amount of reagent, we do not have sufficient separation because we have non-specific labelling, either by the protein or by the fluorochrome. This happens, above all, because the protein-fluorochrome conjugation is not efficient and we have molecules that are not functional, but do cause non-specific binding, which forces us to increase the titre in order to bind the specific cell.
C) If we increase the titre (or optimise it), we can achieve a better separation, but with the risk of increasing non-specific binding due to excess reagent. Separation is not as efficient as with dextran.
It can be used in the following fields
Monitoring the immune response to vaccines by detecting antigen-specific B-lymphocytes; very small populations that are difficult to detect and discriminate with conventional methods, but are not a problem for fluorescent dextran.
Autologous cancer therapy using modified T-cells, which must be detected. This detection is often problematic, either because of the detection protein or because of the number of modified cells and their CAR receptor expression; fluorescent dextrans are able to solve these problems.
It may well be the case that this route is needed for proteins that are difficult to conjugate by conventional methods; in addition, it offers better results for detecting very small populations.
The number and arrangement of antigens incorporated in the fluorescent dextran allow for a very efficient presentation to the cells in order to seek activation, which will be more likely and efficient.
Frequently Asked Questions (FAQ)
Yes, but it must be conjugated with biotin (biotinylated) in order to make use of the biotin-streptavidin system and bind to the dextran.
Yes, any biotinylated molecule is compatible with dextrans, but always titrate the amount of molecule per dextran to optimise the reactive
Yes, there may be exceptions, but it usually works significantly better if it is monobiotinylated, since the conjugation is directed to a site far away from the recognition site. However, the system also works with normal biotinylated
No, no purification is necessary, as the amount that is added to the dextran is titrated so that there is no excess to interfere with the assay.
It is not necessary, but it can be diluted in the desired buffer, taking into account that a stable buffer must be added for the final mixture if it is to be kept diluted for a long time. It is necessary to calculate the volume per test according to the product data sheet (TDS).
Yes, fluorescent dextran has little or no interference, both with other reagents and with different biological samples.