- Productos
- Oncohematología
- Inmunología
- Anticuerpos
- Reactivos individuales
- Determinación de compatibilidad cruzada (FCXM)
- FcεR1
- Subclases Ig
- Reactivos individuales
- Kits
- Solicitar información
- Recursos y soporte
- Anticuerpos
- Exosomas
- Reactivos accesorios
- Software
- Tienda
- Servicios
- Síntesis de Péptidos
- Diseño
- Modificación
- Servicios de Proteínas
- Expresión y purificación
- Liofilización
- Desarrollo de anticuerpos
- Monoclonal
- Policlonal
- Servicios especializados de anticuerpos
- OEM/Producción a granel
- Purificación
- Conjugación
- Servicios de exosomas
- Aislamiento y purificación
- Caracterización
- Síntesis de Péptidos
- Soporte
- Sobre nosotros
- Contacto
BrightStep™: Revolutionizing CAR T-Cell Detection with Superior Flow Cytometry Reagents
As CAR T-cell therapies continue to revolutionize cancer treatment, accurate and efficient detection of these engineered cells has become a critical aspect of the therapeutic process. Immunostep’s innovative BrightStep™ technology presents a game-changing solution for researchers and clinicians, offering unmatched precision and simplicity in identifying CAR T-cells.
▌The Power of CAR T-Cell Therapy
Chimeric Antigen Receptor T-Cell (CAR T-cell) therapy has emerged as a breakthrough in cancer immunotherapy. By modifying a patient’s T-cells to express a receptor specific to an antigen on cancer cells, these engineered cells can effectively target and destroy malignant cells. This targeted approach not only enhances treatment efficacy but also provides a tailored therapeutic option for patients with specific types of cancer, such as B-Cell Acute Lymphoblastic Leukemia (ALL) and Diffuse Large B-Cell Lymphoma (DLBCL).
▌Challenges in CAR T-Cell Detection
The success of CAR T-cell therapy hinges on the precise modification and monitoring of these cells. Traditional reagents used in flow cytometry to identify CAR T-cells face significant limitations, including poor sensitivity and complex, time-consuming protocols. These drawbacks can lead to inaccurate results and hinder the therapeutic process.
▌Introducing BrightStep™ Technology
Immunostep’s BrightStep™ technology addresses these challenges head-on. Our Flow Cytometry Reagent for CAR T-cells offers a single, high-resolution solution that simplifies the detection process. Key benefits of our reagent include:
- High Resolution Identification: BrightStep™ can distinguish CAR T-cells from other cell populations with exceptional clarity, even in challenging samples;
- Simplicity and Efficiency: The reagent’s straightforward protocol requires only a 30-minute incubation at room temperature, with a total protocol time of just one hour. This is significantly faster compared to other commercially available reagents;
- Compatibility and Versatility: Labeled with R-Phycoerythrin (R-PE), our reagent is compatible with any flow cytometer and can be incorporated into panels with over 30 antibodies;
- No Secondary Reagents Needed: Unlike other products that require additional amplification steps, BrightStep™ provides direct detection without the need for secondary reagents.
▌Advantages Over Competitors
Current market alternatives, such as those based on biotinylated proteins, involve indirect staining methods that are not only more costly but also more complex. BrightStep™ overcomes these limitations by offering a direct staining approach that ensures reliable and reproducible results. Our proprietary labeling technique preserves the integrity of the protein, resulting in high specificity and sensitivity.
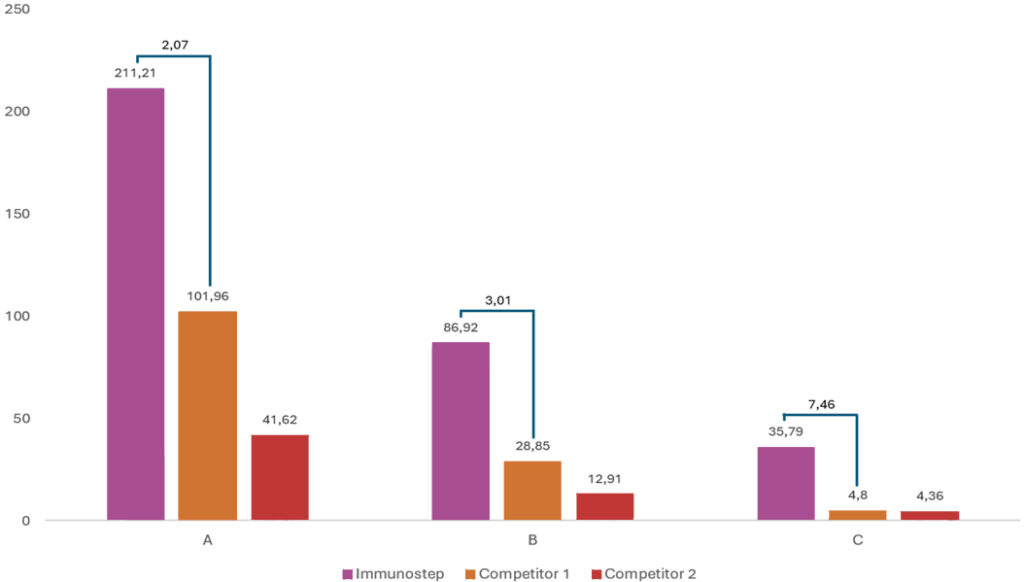
▌Proven Performance
Extensive testing has demonstrated the superior performance of BrightStep™ across various clinical samples, including those with low CAR expression. Our reagent consistently provides a higher Stain Index (SI), a crucial metric for distinguishing positive CAR T-cells from negative populations. This makes BrightStep™ an indispensable tool for both quality control during the modification process and post-infusion monitoring.
▌Future Developments
Immunostep is committed to advancing the field of CAR T-cell therapy. We are actively working on developing reagents for other CAR targets, such as BCMA for Multiple Myeloma, and exploring additional fluorescent tags to enhance compatibility with existing cytometry panels.
▌Conclusion
BrightStep™ sets a new standard for CAR T-cell detection, combining innovation with practicality to support the ongoing evolution of cancer immunotherapy. By simplifying the detection process and ensuring high-resolution results, Immunostep’s reagent empowers researchers and clinicians to achieve more reliable outcomes, ultimately improving patient care.
For more detailed information, check these links: